
Our laboratory focuses on two neuroendocrine systems: The hypothalamic-pituitary-adrenal (HPA) axis and the hypothalamic-pituitary-gonadal (HPG) axis.
The health of mammals depends on their ability to maintain and/or restore homeostasis when faced with threats to the consistency of their "milieu interieur". Understanding the mechanisms that regulate homeostasis in healthy organisms, and the way in which their malfunction may contribute to pathologies, is therefore central to a variety of health issues. Our laboratory has a long-standing interest in uncovering the mechanisms underlying the body's response to challenges, i.e., how the central nervous system (CNS) organizes neuroendocrine responses in the brain and the periphery when it perceives the occurrence of a stressor. The HPA axis is central to the organization of this response in that neuroendocrine actions driven by the HPA axis are the primary means by which an organism responds to threats (perceived or real). The threats that we study include those that are relevant to humans, in particular, physico-emotional challenges, immune challenges and drugs such as alcohol. By determining changes in neuronal and hormonal activity following stressful stimuli, we seek to describe the components and pathways integral to HPA axis function.
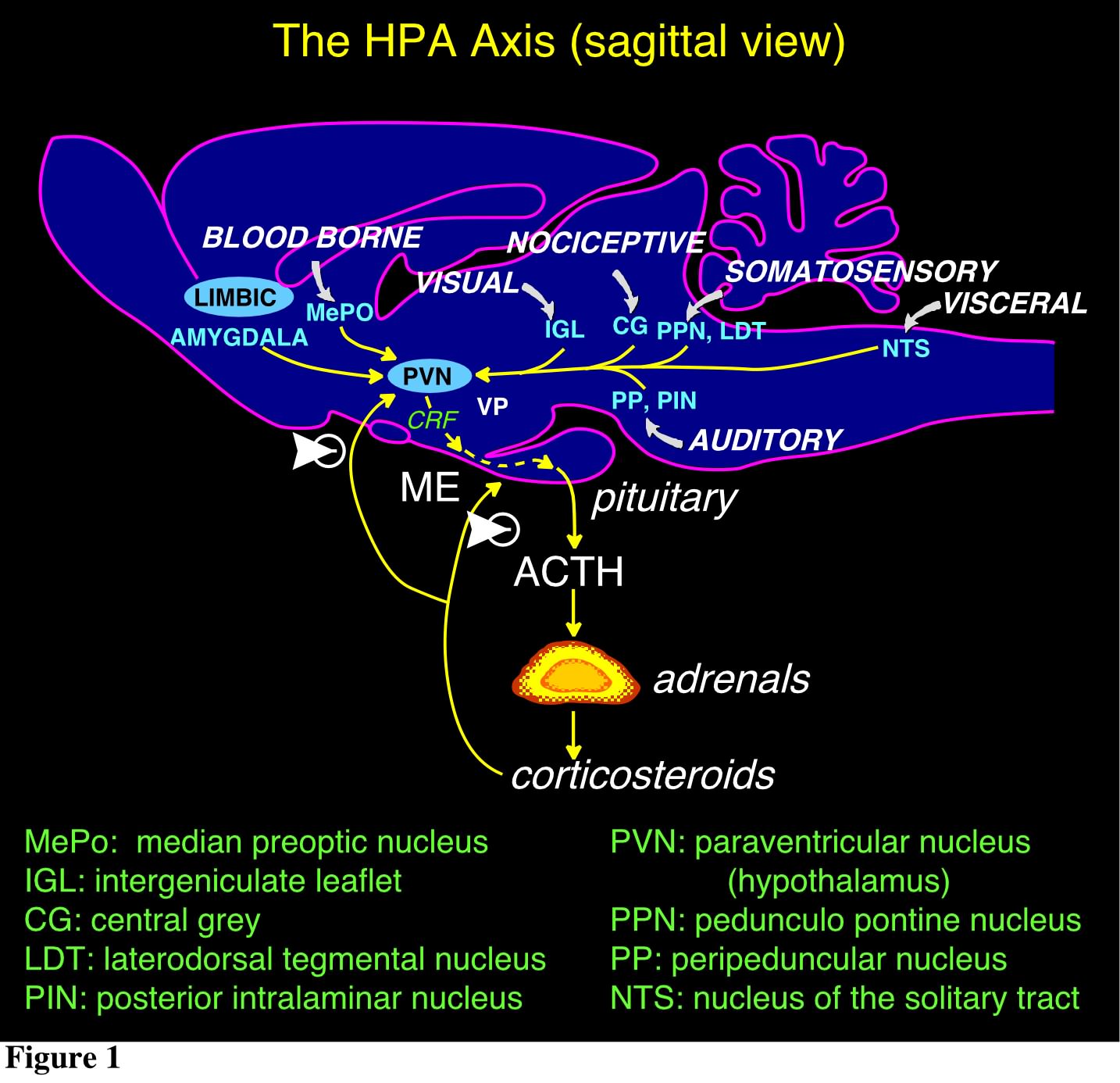
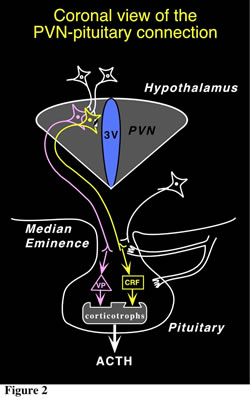 The hypothalamus is a major integrating center for receiving messages from different centers and converting them to hormonal signals, via the control of the pituitary gland and by neural pathways. The main CNS nucleus involved in the regulation of the pituitary-adrenal axis is the paraventricular nucleus (PVN) of the hypothalamus. The PVN is the principal CNS source of the 41-amino acid peptide corticotropin-releasing factor (CRF), which is the major physiological regulator of pituitary adrenocorticotropin hormone (ACTH) secretion, and of vasopressin (VP), an octapeptide which interacts with CRF to promote ACTH release. These neurons represent the final output component of a neuronal network that integrates multiple environmental and internal factors to regulate the body's response to homeostasis threats. Thus, the PVN receives a diverse afferent supply which includes all major routes conveying sensory information, as well as pathways originating from the limbic forebrain and other hypothalamic cell groups (Fig. 1). The CRF and VP hypophysiotropic neurons from the PVN project to the external zone of the median eminence (ME) and release peptides into a specialized capillary network, the portal system. The anterior pituitary (or adenohypophysis) is vascularized by these hypophysial portal vessels, that arise from these ME capillary beds. Within the anterior pituitary, CRF interacts with a specific G protein-coupled receptor (CRF-R1) on the corticotrope cell surface, triggering a cascade of enzymatic reactions that begin with stimulation of adenylate cyclase and ultimately regulate proopiomelanocortin (POMC) gene expression and release of POMC-derived peptides in the fasciculata of the adrenal cortex (cortisol in humans; corticosterone in rats and mice; Fig. 2). In a classical endocrine feedback manner, these steroids inhibit the synthesis and secretion of CRF and VP within the hypothalamus and of POMC-derived peptides in the pituitary (Fig. 1).
The hypothalamus is a major integrating center for receiving messages from different centers and converting them to hormonal signals, via the control of the pituitary gland and by neural pathways. The main CNS nucleus involved in the regulation of the pituitary-adrenal axis is the paraventricular nucleus (PVN) of the hypothalamus. The PVN is the principal CNS source of the 41-amino acid peptide corticotropin-releasing factor (CRF), which is the major physiological regulator of pituitary adrenocorticotropin hormone (ACTH) secretion, and of vasopressin (VP), an octapeptide which interacts with CRF to promote ACTH release. These neurons represent the final output component of a neuronal network that integrates multiple environmental and internal factors to regulate the body's response to homeostasis threats. Thus, the PVN receives a diverse afferent supply which includes all major routes conveying sensory information, as well as pathways originating from the limbic forebrain and other hypothalamic cell groups (Fig. 1). The CRF and VP hypophysiotropic neurons from the PVN project to the external zone of the median eminence (ME) and release peptides into a specialized capillary network, the portal system. The anterior pituitary (or adenohypophysis) is vascularized by these hypophysial portal vessels, that arise from these ME capillary beds. Within the anterior pituitary, CRF interacts with a specific G protein-coupled receptor (CRF-R1) on the corticotrope cell surface, triggering a cascade of enzymatic reactions that begin with stimulation of adenylate cyclase and ultimately regulate proopiomelanocortin (POMC) gene expression and release of POMC-derived peptides in the fasciculata of the adrenal cortex (cortisol in humans; corticosterone in rats and mice; Fig. 2). In a classical endocrine feedback manner, these steroids inhibit the synthesis and secretion of CRF and VP within the hypothalamus and of POMC-derived peptides in the pituitary (Fig. 1).
Our interest in neuroendocrine systems includes the control of reproductive physiology by the HPG axis. We examine how the actions of and communication among axial components are integrated to result in normal reproductive function. We also test the effect of various factors (e.g., chemical, immune, and psychological stressors) on HPG axis activity, and investigate the mechanisms that mediate their effect. A recent addition to our research is the elucidation of a novel hypothalamic-testicular pathway that does not include the pituitary, and for which we propose a role in so-far unexplained stress-induced reproductive disorders.
Our interest in neuroendocrine systems includes the control of reproductive physiology by the HPG axis. We examine how the actions of and communication among axial components are integrated to result in normal reproductive function. We also test the effect of various factors (e.g., chemical, immune, and psychological stressors) on HPG axis activity, and investigate the mechanisms that mediate their effect. A recent addition to our research is the elucidation of a novel hypothalamic-testicular pathway that does not include the pituitary, and for which we propose a role in so-far unexplained stress-induced reproductive disorders.
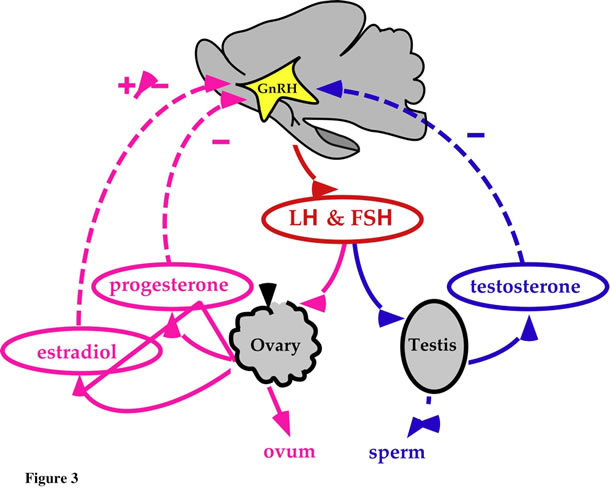
The HPG axis is composed of the hypothalamus and its neural connections with the rest of the brain, the pituitary, and the testis (male) or ovary (female) (Fig. 3). The classic view of this axis is that the anterior hypothalamus and preoptic area represent the regions that are responsible for the synthesis of the peptide gonadotropin-releasing hormone (GnRH), the primary regulator of the pituitary gonadotrophs. Axons arising from these hypothalamic nuclei project toward the medial basal hypothalamus and into the outer zone of the median eminence. From the ME, GnRH reaches the anterior pituitary via portal veins and stimulates gonadotropic cells to release luteinizing hormone (LH) and follicle stimulating hormone (FSH) into the general circulation. LH and FSH bind to receptors in the ovary and testis and regulate gonadal function by stimulating sex steroid production and gametogenesis. In the male, LH causes testosterone to be produced from the Leydig cells of the testes. LH in combination with FSH is required for maturation of spermatozoa. FSH stimulates testicular growth and increases production of androgen-binding protein by Sertoli cells. Androgen-binding protein concentrates testosterone near the sperm, enabling normal spermatogenesis. In the female, LH stimulates ovarian production of estrogen and progesterone. An LH surge midway in the cycle causes ovulation, and sustained LH secretion stimulates the corpus luteum to produce progesterone. FSH exerts primary control over development of the ovarian follicle, and FSH and LH are responsible for follicular secretion of estrogen.
In addition to this classical view, we have recently proposed the existence of a neural pathway (not represented in Fig. 3) influenced by brain catecholamines, that rapidly blocks testicular responsiveness to gonadotropins.
© 2012 Salk Institute for Biological Studies
10010 North Torrey Pines Road, La Jolla, CA 92037 | 858.453.4100 | webmaster@salk.edu
